

The electrons spin in orbits around the outside of the nucleus. The nucleus is made up of the protons and neutrons. But the nucleus is very hard to split, meaning most atoms are around for a long time.Īt the center of the atom is the nucleus. They can change and undergo chemical reactions, sharing electrons with other atoms. There are 92 natural elements and up to 118 when you count in man-made elements.Ītoms last a long time, in most cases forever. Each different kind of atom makes up an element. There are different kinds of atoms based on the number of electrons, protons, and neutrons each atom contains.
Suffice it to say that the number is trillions and trillions (and then some more). There are so many atoms in a single human body we won't even try to write the number here. It takes a lot of atoms to make up anything. Atoms fit together with other atoms to make up matter. The basic particles that make up an atom are electrons, protons, and neutrons. Atoms are extremely small and are made up of a few even smaller particles.
#ATOM DEFINITION FREE#
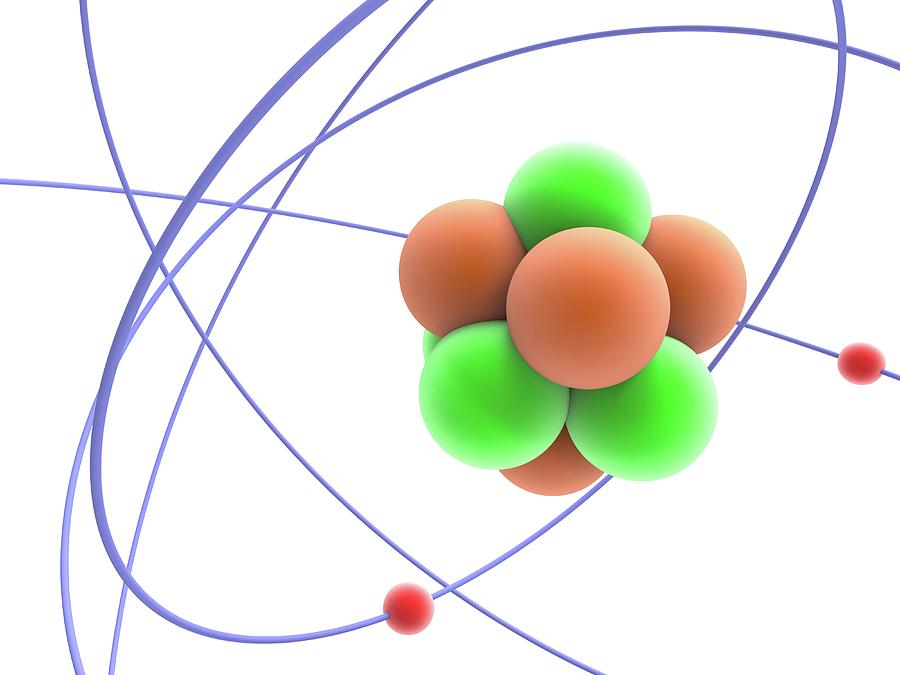
This document is free of copyright and can be reproduced for educational purposes.The atom is the basic building block for all matter in the universe. Nuclear Regulatory Commission is an independent federal government agency responsible for regulating the commercial use of nuclear materials. Just as several atoms make up a molecule, many molecules make up a chemical. These bonds allow for the formation of molecules, combinations of atoms (including those of different elements). If an atom has a different number of electrons and protons, it is called an ion.Īn important principle to know is electrons may be transferred from one atom to another or even shared between atoms (allowing atoms to bind together). An atom is considered to be electrically neutral if it has an equal number of protons and electrons. Remember, electrons are negatively-charged and are attracted to the positively-charged protons in the nucleus. The nucleus of an atom is surrounded by a cloud of electrons. Generally speaking, atoms with roughly matching numbers of protons and neutrons are more stable against decay. While radioactive decay can occur in a variety of ways, it is, simply put, the process by which unstable atoms break down, releasing particles (and energy). This is important to the NRC because the number of neutrons relative to the protons determines the stability of the nucleus, with certain isotopes undergoing radioactive decay. The number of neutrons determines what isotope an atom is. Now, while the protons are the same in an element, the number of neutrons may vary from atom to atom. In other words, one element cannot be transformed into another (again, with the exception of nuclear reactions). An element, like hydrogen, oxygen or iron, is a substance that cannot be broken down-outside of a nuclear reaction-into anything else. Two atoms with an identical number of protons in their nuclei belong to the same element. The number of protons in the nucleus also defines in large part the characteristics of an atom-is it a gas or a metal, for example. The number of protons in the nucleus, known as the "atomic number," primarily determines where that atom fits on the Periodic Table. The nucleus (or center) of an atom is made up of protons and neutrons. Likewise, just as when you experience resistance trying to push the same ends of two magnets together, protons are repelled from other protons and electrons are repelled from other electrons. So, much like opposite ends of a magnet, protons and electrons are attracted to each other. A fundamental rule is that particles with the same charge are repulsed from each other, while particles with opposite charges are attracted to each other. Neutrons, on the other hand, don't have a charge. Two of the subatomic particles have electrical charges: protons have a positive charge while electrons have a negative charge. There are three subatomic particles: protons, neutrons and electrons. Given that these particles make up atoms, they are often referred to as subatomic particles. While its name originally referred to a particle that couldn't be divided any more-the smallest thing possible-we now know that each atom is generally made up of smaller particles. Anything that has a mass-in other words, anything that occupies space-is composed of atoms. The atom is considered the basic building block of matter. Printable Version The Nuclear Regulatory Commission's Science 101: What is an Atom?


 0 kommentar(er)
0 kommentar(er)
